5 Ways Sulfuric Acid is Used in Spectroscopy

Introduction to Sulfuric Acid in Spectroscopy
Sulfuric acid, a highly corrosive and dense liquid, is widely used in various industrial and laboratory applications. One of its significant uses is in spectroscopy, a scientific technique used to measure the interaction between matter and electromagnetic radiation. Spectroscopy helps analyze the composition and properties of materials, and sulfuric acid plays a crucial role in this process. In this article, we will explore five ways sulfuric acid is used in spectroscopy.
1. Sample Preparation in Infrared Spectroscopy
Infrared (IR) spectroscopy is a technique used to identify the molecular structure of a sample by analyzing the absorption of infrared radiation. Sulfuric acid is often used as a solvent to prepare samples for IR spectroscopy. It is particularly useful for analyzing organic compounds, as it can dissolve a wide range of substances. By dissolving the sample in sulfuric acid, it becomes possible to obtain a clear IR spectrum, which can be used to identify the molecular structure of the compound.
🔥 Note: When handling sulfuric acid, it is essential to wear protective gloves and eyewear, as it can cause severe burns and eye damage.
2. Matrix-Assisted Laser Desorption/Ionization (MALDI) Mass Spectrometry

MALDI mass spectrometry is a technique used to analyze the mass-to-charge ratio of ions in a sample. Sulfuric acid is used as a matrix in MALDI to facilitate the ionization of the sample. The acid helps to create a uniform matrix that allows for efficient ionization and detection of the sample’s components. This technique is commonly used in the analysis of biomolecules, such as proteins and peptides.
| Matrix | Sample | Ionization |
|---|---|---|
| Sulfuric acid | Biomolecules (proteins, peptides) | UV laser |
3. Acid Digestion in Inductively Coupled Plasma (ICP) Spectroscopy
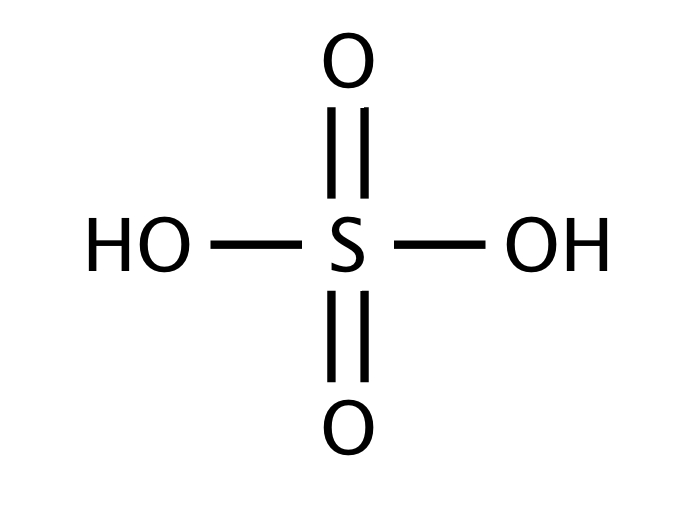
ICP spectroscopy is a technique used to analyze the elemental composition of a sample. Sulfuric acid is often used to digest the sample, breaking it down into its constituent elements. The acid helps to release the elements from the sample matrix, allowing for their detection and quantification by ICP spectroscopy. This technique is commonly used in the analysis of environmental and geological samples.
🔄 Note: When performing acid digestion, it is essential to use a fume hood to prevent exposure to toxic fumes.
4. Solvent in Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a technique used to analyze the molecular structure of a sample by measuring the interaction between nuclear spins and magnetic fields. Sulfuric acid is sometimes used as a solvent in NMR spectroscopy, particularly for analyzing organic compounds. The acid helps to dissolve the sample and provide a clear NMR spectrum, which can be used to identify the molecular structure of the compound.
5. Catalyst in Raman Spectroscopy

Raman spectroscopy is a technique used to analyze the vibrational modes of molecules. Sulfuric acid is sometimes used as a catalyst in Raman spectroscopy to enhance the signal-to-noise ratio of the spectrum. The acid helps to increase the intensity of the Raman signal, allowing for more accurate analysis of the sample’s molecular structure.
The use of sulfuric acid in spectroscopy has significantly contributed to the advancement of analytical techniques. Its ability to dissolve and break down samples, facilitate ionization, and enhance spectral signals has made it an essential reagent in many spectroscopic applications. However, it is crucial to handle sulfuric acid with care, as it can be hazardous if not handled properly.
In summary, sulfuric acid plays a vital role in various spectroscopic techniques, including IR spectroscopy, MALDI mass spectrometry, ICP spectroscopy, NMR spectroscopy, and Raman spectroscopy. Its ability to dissolve and break down samples, facilitate ionization, and enhance spectral signals has made it an essential reagent in many spectroscopic applications.
What is the primary use of sulfuric acid in spectroscopy?

+
The primary use of sulfuric acid in spectroscopy is as a solvent to prepare samples for analysis.
What is the role of sulfuric acid in MALDI mass spectrometry?

+
Sulfuric acid is used as a matrix in MALDI to facilitate the ionization of the sample.
What safety precautions should be taken when handling sulfuric acid?

+
When handling sulfuric acid, it is essential to wear protective gloves and eyewear, and to work in a well-ventilated area to prevent exposure to toxic fumes.